Introduction to Dehydration of Alcohol Mechanism
Have you ever wondered why drinking alcohol makes you feel dehydrated or what happens when it is used in industrial reactions? The Dehydration of Alcohol Mechanism is a fascinating concept that connects the worlds of chemistry and health. On the one hand, it is an important reaction in organic chemistry that transforms alcohol into an alkane. On the other hand, it reminds us how alcohol consumption can disturb the body’s hydration balance.
In this article, we will delve deeper into the science of the mechanism of alcohol dehydration, explore its practical applications and discuss its impact on human health.
Table of Contents
The Chemistry Behind the Dehydration of Alcohol Mechanism
What is the Dehydration of Alcohol Mechanism?
Basically, the alcohol dehydration mechanism refers to the chemical reaction in which a water molecule is separated from an alcohol molecule to form an alkene. This process generally requires an acid catalyst and heat to proceed effectively.
Overview of Alcohol Molecules
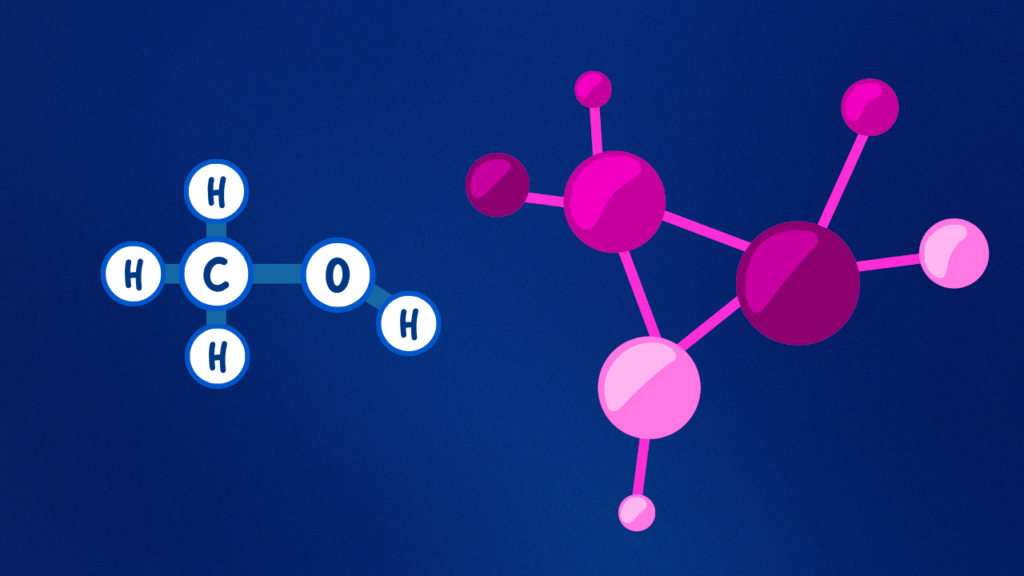
Alcohols are organic compounds that have a hydroxyl group (-OH) attached to a carbon atom. Their structure determines how easily they undergo dehydration. Primary, secondary, and tertiary alcohols have different reactions in this mechanism.
The Step-by-Step Dehydration Process
Alcohols are organic compounds that have a hydroxyl group (-OH) attached to a carbon atom. Their structure determines how easily they undergo dehydration. Primary, secondary, and tertiary alcohols have different reactions in this mechanism.
- Protonation of Alcohol: The reaction begins when the hydroxyl group of the alcohol is protonated by an acid catalyst such as sulfuric acid, making it a better leaving group.
- Formation of Carbocation: Loss of water leads to the formation of a positively charged intermediate called a carbocation.
- Elimination to form an Alkene: Finally, a proton is removed from a neighbouring carbon atom, resulting in the formation of a double bond (alkene).
Catalysts and Reaction Conditions
This reaction requires an acidic catalyst such as H2SO4 or H3PO4 and high temperatures. The type of alcohol and the conditions also affect the efficiency and outcome of the reaction.
Industrial Applications of Alcohol Dehydration
Production of Ethylene and Other Alkenes
The dehydration of ethylene from ethanol is a classic example. Ethylene is an important compound used in the production of plastics, synthetic fibers, and other industrial materials. Uses in biofuel synthesis.
Alcohol dehydration plays an important role in the sustainable production of biofuels, reducing dependence on fossil fuels.
Alcohol and Its Effects on the Human Body

How Alcohol Causes Dehydration in the Body
When we talk about the dehydrating mechanisms of alcohol, the mind often turns to chemistry labs and industrial reactions. But there’s another kind of “mechanism” that happens when alcohol enters our bodies – and it’s much closer to home. Have you ever woken up with a pounding headache, dry mouth and thirst after a night out? This is your body telling you it’s dehydrated. But why does alcohol do this? Let’s explore the science and effects behind this phenomenon.
Alcohol’s Dual Nature: Friend and Foe
Alcohol or ethanol is the main ingredient in beer, wine, and spirits. Although it is often drunk for relaxation and social bonding, it also has its disadvantages, especially in how it affects our body’s hydration levels. Unlike water, which replenishes our fluids, alcohol actively works against our hydration efforts.
When alcohol is consumed, it reacts with multiple systems in the body, and unfortunately, most of these interactions result in water loss. Let’s understand the main mechanisms behind why alcohol causes dehydration.
The Diuretic Effect: The Culprit Behind Dehydration
The main reason alcohol causes dehydration is its diuretic effect. A diuretic is a substance that increases urine production, causing your body to excrete more water than it takes in. When alcohol enters your system, it affects a hormone called antidiuretic hormone (ADH).
- What is ADH? ADH, also known as vasopressin, is a hormone produced by the brain that helps your kidneys manage the amount of water in your body. Think of it as the gatekeeper that tells your kidneys whether to hold on to water or let it go.
- How Alcohol Interferes with ADH When you drink alcohol, it suppresses the production of ADH. Without enough ADH, your kidneys get the green signal to release more water through urine. Essentially, you go to the bathroom far more often than normal. With each go, your body is losing water, electrolytes, and essential minerals that keep you hydrated.
This mechanism is very similar to the chemical dehydration alcohol mechanism, where water is removed from the alcohol molecules during the reaction. In the body, alcohol consumption triggers a similar water-loss process, but instead of forming an alkenes, it reduces your hydration levels.
Electrolyte Imbalances and Their Role in Dehydration
Water isn’t the only thing you lose when alcohol works its magic on your kidneys. Along with urine, your body also expels electrolytes like sodium, potassium, and magnesium. These are important for maintaining hydration, nerve function, and muscle health.
- Why Electrolytes Matter Electrolytes act like tiny electrical signals in your body. They help regulate fluid balance, muscle contractions, and even your heartbeat. Losing too many electrolytes due to dehydration caused by alcohol can leave you feeling tired, dizzy, and weak.
- The Vicious Cycle As your body loses electrolytes, it struggles to retain water. This creates a vicious cycle where dehydration worsens, leading to symptoms such as dry mouth, headaches, and dizziness.
Alcohol’s Impact on the Kidneys
Your kidneys are the superheroes of your body’s hydration system. They filter blood, remove waste, and regulate water levels. But when alcohol gets in, it upsets this balance.
Overburdening the Kidneys
Drinking alcohol puts the kidneys to work harder, which excretes not only alcohol but also excess water and electrolytes. If binge drinking becomes a habit, this increased load can cause long-term damage.
Reduced Water Reabsorption
Normally, your kidneys reabsorb some of the water that passes through them to keep your body hydrated. But when ADH levels are lowered due to alcohol, this reabsorption process slows down, leading to greater fluid loss.
How Dehydration Affects the Body’s Systems
Dehydration from alcohol not only makes you feel thirsty – it also has a profound effect on a variety of other body functions:
The Brain
Dehydration reduces the fluid surrounding the brain, which can cause headaches and difficulty concentrating. Feeling foggy after drinking? It’s partly due to your brain demanding water.
The Skin
Alcohol dehydrates your skin, making it drier and less elastic. This can make you look tired and cause fine lines and wrinkles to appear on the skin.
The Heart
Dehydration causes your heart to work harder to pump blood, which can increase your heart rate and cause fatigue.
.
The Digestive System
Alcohol irritates the stomach lining and slows digestion, which can increase the dehydrating effects, especially if you’re not drinking enough water with the alcohol
.
Symptoms of Alcohol-Induced Dehydration

How do you know if your body is dehydrated after drinking alcohol? Here are some signs:
Dry Mouth
A lack of saliva is one of the earliest signs of dehydration. You may feel like your mouth is dry, no matter how much water you drink.
Headaches
Dehydration caused by alcohol can reduce blood flow to the brain, which can lead to terrible hangover headaches.
Fatigue
Loss of water and electrolytes reduces your energy levels, making you feel sluggish.
Dizziness
A drop in blood pressure due to dehydration may cause you to feel dizzy or lightheaded.
Dark Urine.
If the colour of your urine is darker than normal, it is a clear sign that your body is lacking fluids.
Factors That Worsen Alcohol-Induced Dehydration
Not all beverages are the same when it comes to dehydration. Factors such as alcohol content, type of beverage, and your own hydration habits play a role:
- High-Alcohol Content: Beverages with a high alcohol percentage, such as spirits, have a strong diuretic effect
- Mixers :Mixers containing sugar can make dehydration worse by increasing water loss.
- Drinking on an Empty Stomach: Without food to slow down alcohol absorption, its diuretic effect works faster.
- Skipping Water: Not drinking water with alcohol increases the problem of dehydration.
Can the Damage Be Prevented?

Although the dehydration mechanism caused by alcohol cannot be completely avoided, there are some ways to reduce its effects:
- Hydrate Beforehand: Drinking water before drinking alcohol can help your body get enough water.
- Alternate Drinks: Drink water or electrolyte-rich beverages with alcohol to maintain balance.
- Choose Wisely: Choose low-alcohol beverages and avoid sugary cocktails.
- Eat While Drinking: Eating a balanced meal can slow alcohol absorption and reduce its diuretic effect.
Lessons from Chemistry: How to Stay Hydrated
While understanding the dehydration mechanisms of alcohol in the lab helps optimize chemical reactions, understanding how alcohol dehydrates your body can help you stay ahead of its effects. By staying hydrated and being mindful of your drinking habits, you can enjoy alcohol without suffering its worst consequences.
Tips to Prevent Alcohol-Induced Dehydration

Hydration Strategies While Drinking Alcohol
- Drinking water with alcohol
- Drink low -alcohol beverages
Foods and Drinks to Restore Balance
- To replace lost nutrients, consume electrolyte-rich foods like bananas or hydrating drinks like coconut water. Drink low -alcohol beverages
Conclusion
The Dehydration of Alcohol Mechanism is more than just a chemistry term—it’s a reminder of how alcohol affects our hydration and overall health. From suppressing ADH to depleting electrolytes, alcohol’s diuretic effect has powerful effects on the body. By understanding the science and taking proactive steps, you can minimize dehydration and enjoy a healthier relationship with alcohol
- You mignt be interested in reading this post as well
- How to stay Healthy & Fit Naturally and Embrace Wellness
FAQs for The Dehydration of Alcohol Mechanism and its effect on Health
Q1. What is the dehydration of alcohol mechanism in simple terms?
A:It’s a chemical process where water is removed from an alcohol molecule to create an alkene.
Q2. How does alcohol consumption cause dehydration in the body?
A:Alcohol increases urine production, leading to fluid and electrolyte loss.
Q3.Can dehydration from alcohol be prevented?
A:Yes, by drinking water alongside alcohol and consuming electrolyte-rich foods.
Q4.Why is the dehydration of alcohol mechanism important in industry?
A:It’s crucial for producing materials like ethylene, which are used in plastics and biofuels.
Q5. What are some health risks of alcohol-induced dehydration?
A:Dehydration can cause fatigue, headaches, electrolyte imbalances, and cognitive difficulties.


